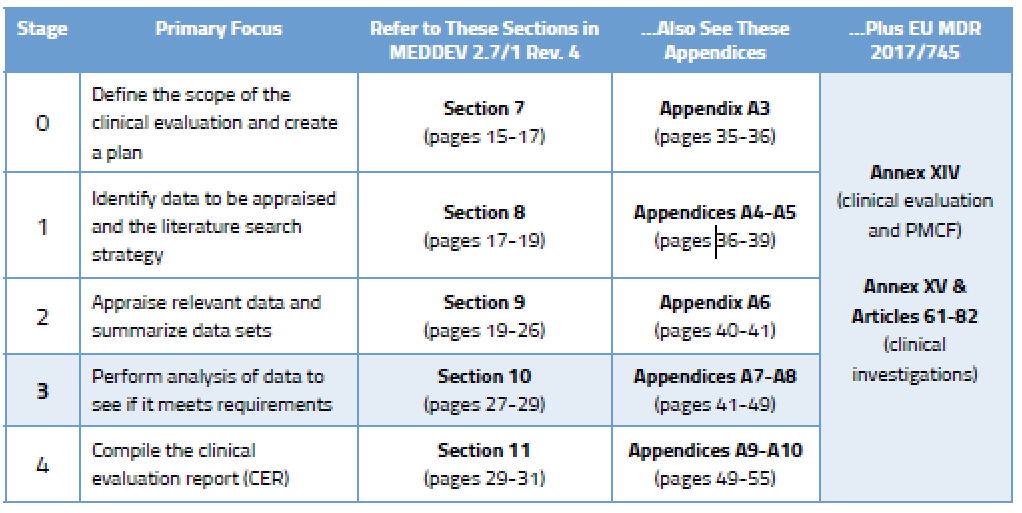
Software as a Medical Device (SAMD): Clinical Evaluation - Guidance for Industry and Food and Drug Administration Staff

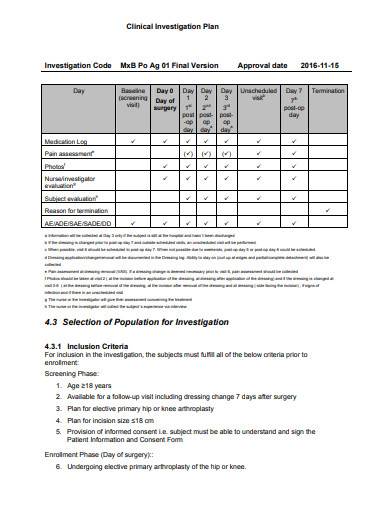
ANSI/AAMI/ISO 14155-2:2003 - Clinical investigation of medical devices for human subjects - Part 2: Clinical investigation plans

Improved clinical investigation and evaluation of high-risk medical devices: the rationale and objectives of CORE–MD (Coordinating Research and Evidence for Medical Devices) in: EFORT Open Reviews Volume 6 Issue 10 (2021)
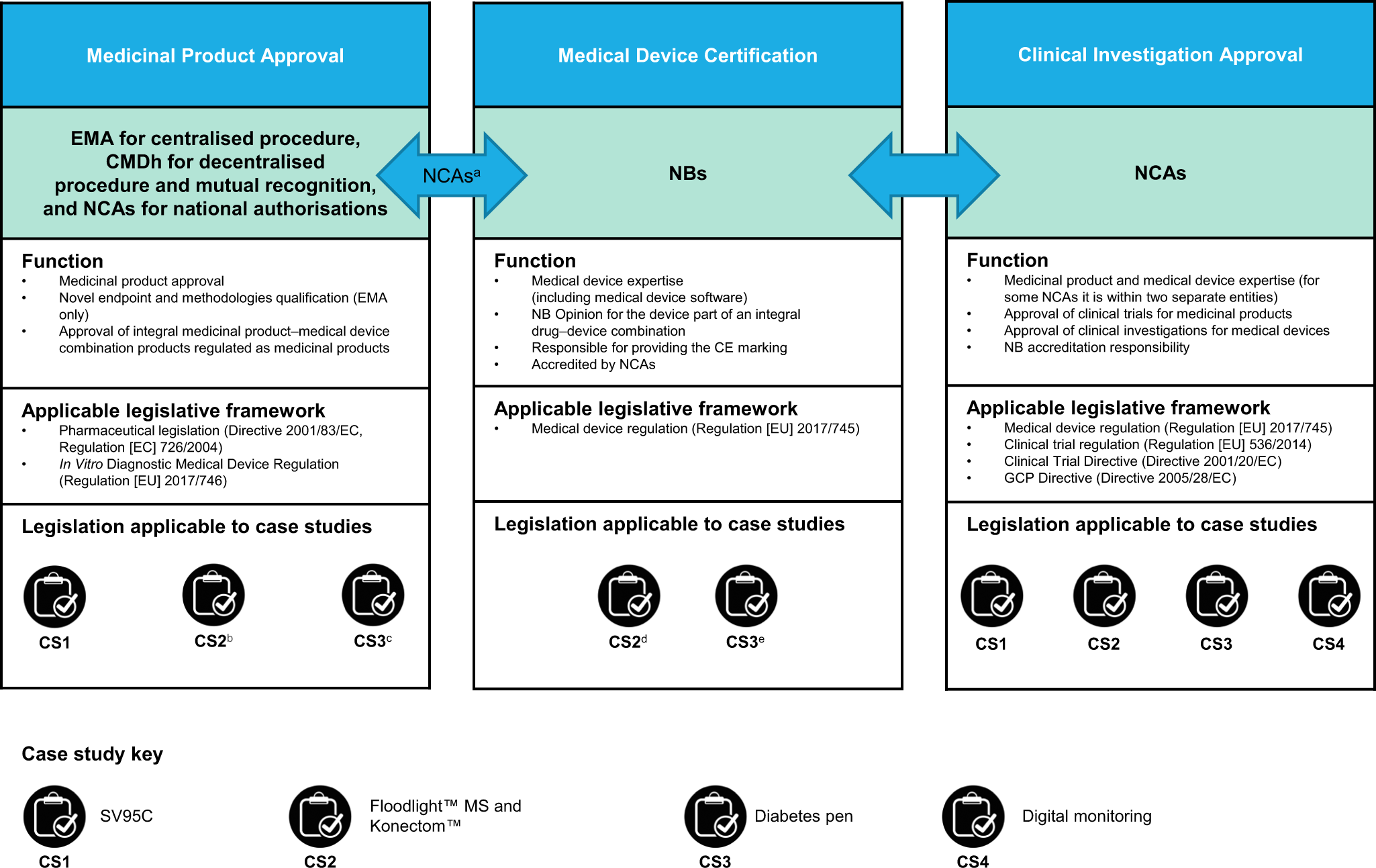
Evolving regulatory perspectives on digital health technologies for medicinal product development | npj Digital Medicine
FDA Decisions for Investigational Device Exemption Clinical Investigations - Guidance for Sponsors, Clinical Investigators, Inst

BS EN ISO 14155-2:2009 - Clinical investigation of medical devices for human subjects. Clinical investigation plans (British Standard)