Impact of the ICH E9 Guideline Statistical Principles for Clinical Trials on the Conduct of Clinical Trials in Japan

TUTORIAL on ICH E9 and Other Statistical Regulatory Guidance. Session 1: ICH E9 and E10. PSI Conference, May PDF Free Download

Early phase clinical trials extension to guidelines for the content of statistical analysis plans | The BMJ
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMON
ICH Topic E 9 Statistical Principles for Clinical Trials NOTE FOR GUIDANCE ON STATISTICAL PRINCIPLES FOR CLINICAL TRIALS

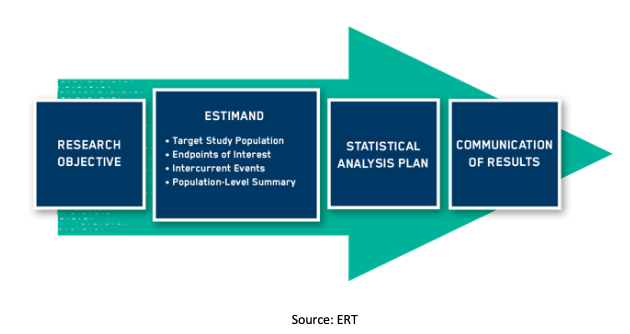
Book 13: 2022 FDA Guidance on Clinical Study Reports and Statistical P – Clinical Research Resources, LLC

International - ICH E9 (R1) addendum on estimands and sensitivity analysis in clinical trials to the guideline on statistical principles for clinical trials - RIS.WORLD
1097-0258(19990815)18:15%3C1903::aid-sim188%3E3.0.co;2-f.fp.png)
Statistical principles for clinical trials (ICH E9): an introductory note on an international guideline - Lewis - 1999 - Statistics in Medicine - Wiley Online Library

ICH E9 guideline 'Statistical principles for clinical trials': a case study Response to A. Phillips and V. Haudiquet - Brown - 2003 - Statistics in Medicine - Wiley Online Library
![Seven steps for statistical success in clinical trials [Good clinical practice guidelines] Part 1 – ACTA – Australian Clinical Trials Alliance Seven steps for statistical success in clinical trials [Good clinical practice guidelines] Part 1 – ACTA – Australian Clinical Trials Alliance](https://clinicaltrialsalliance.org.au/wp-content/uploads/2020/10/James-Matcham-Photo_2020-500x334.jpg)