
COVID-19-related research in Africa: a cross-sectional review of the International Clinical Trial Registration Platform (ICTRP) – ScienceOpen

World Health Organization (WHO) on Twitter: "WHO International Clinical Trials Registry Platform (ICTRP) lists now 27 clinical trials on #2019nCoV. 24 of these are listed in #China. Search will be updated and
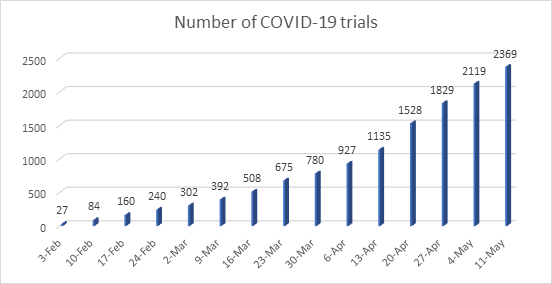
The WHO International Clinical Trials Registry Platform: Providing global clinical trial information to all - On Medicine

What does ICTRP mean? - Definition of ICTRP - ICTRP stands for International Clinical Trials Registry Platform. By AcronymsAndSlang.com

In the name of God 1 Dr. Davood Khalili. International Clinical Trials Registry Sites ClinicalTrials.gov The WHO Registry Network International Clinical. - ppt download

A Review of Ginseng Clinical Trials Registered in the WHO International Clinical Trials Registry Platform. - Abstract - Europe PMC

SciELO - Brasil - Practical and conceptual issues of clinical trial registration for Brazilian researchers Practical and conceptual issues of clinical trial registration for Brazilian researchers

COVID-19 Therapeutics for Low- and Middle-Income Countries: A Review of Candidate Agents with Potential for Near-Term Use and Impact in: The American Journal of Tropical Medicine and Hygiene Volume 105 Issue 3 (

Global trends in chronic thromboembolic pulmonary hypertension clinical trials and dissemination of results - McInnis - 2021 - Pulmonary Circulation - Wiley Online Library

Survey of clinical trials in children in WHO International Clinical Trials Registry Platform | The 20th Cochrane Colloquium

SciELO - Saúde Pública - Analysis of registered cancer clinical trials in Latin America and the Caribbean, 2007-2013 Analysis of registered cancer clinical trials in Latin America and the Caribbean, 2007-2013

SciELO - Saúde Pública - Analysis of registered cancer clinical trials in Latin America and the Caribbean, 2007-2013 Analysis of registered cancer clinical trials in Latin America and the Caribbean, 2007-2013
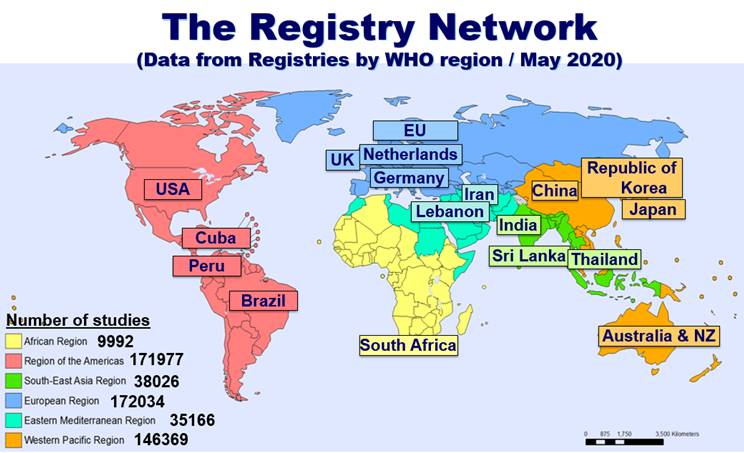
The WHO International Clinical Trials Registry Platform: Providing global clinical trial information to all - On Medicine

Studies registered in non-ClinicalTrials.gov accounted for an increasing proportion of protocol registrations in medical research - Journal of Clinical Epidemiology












