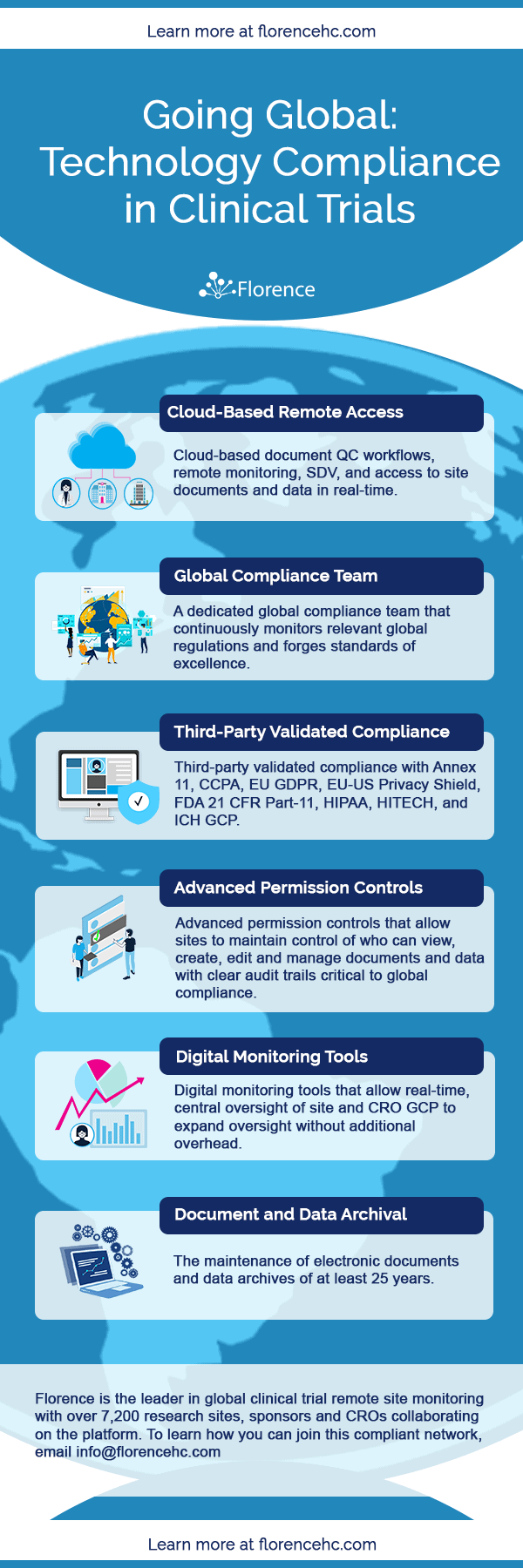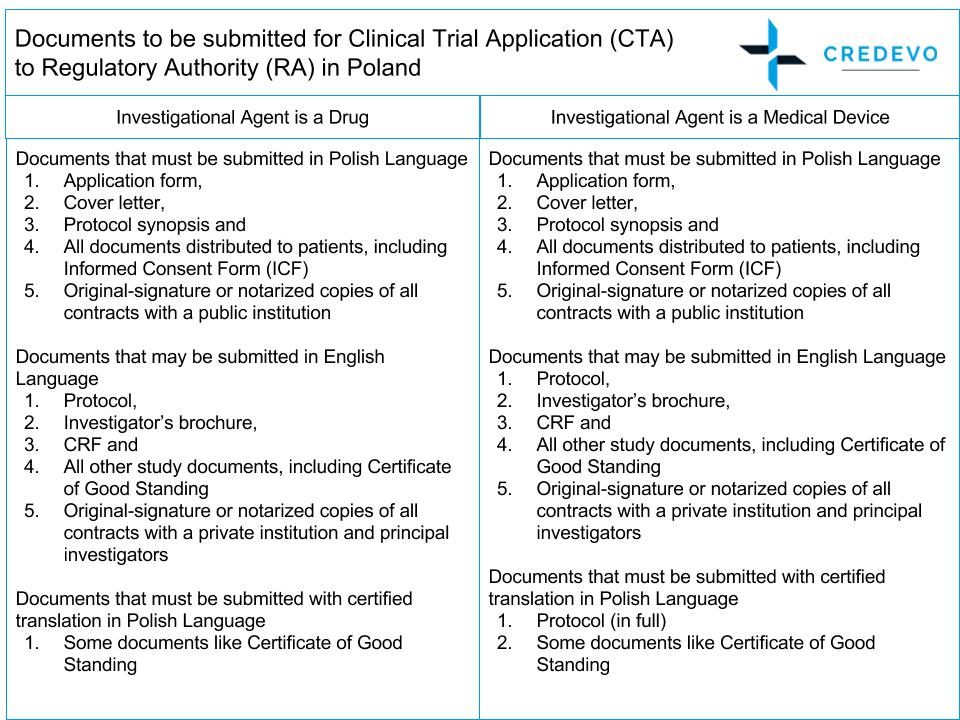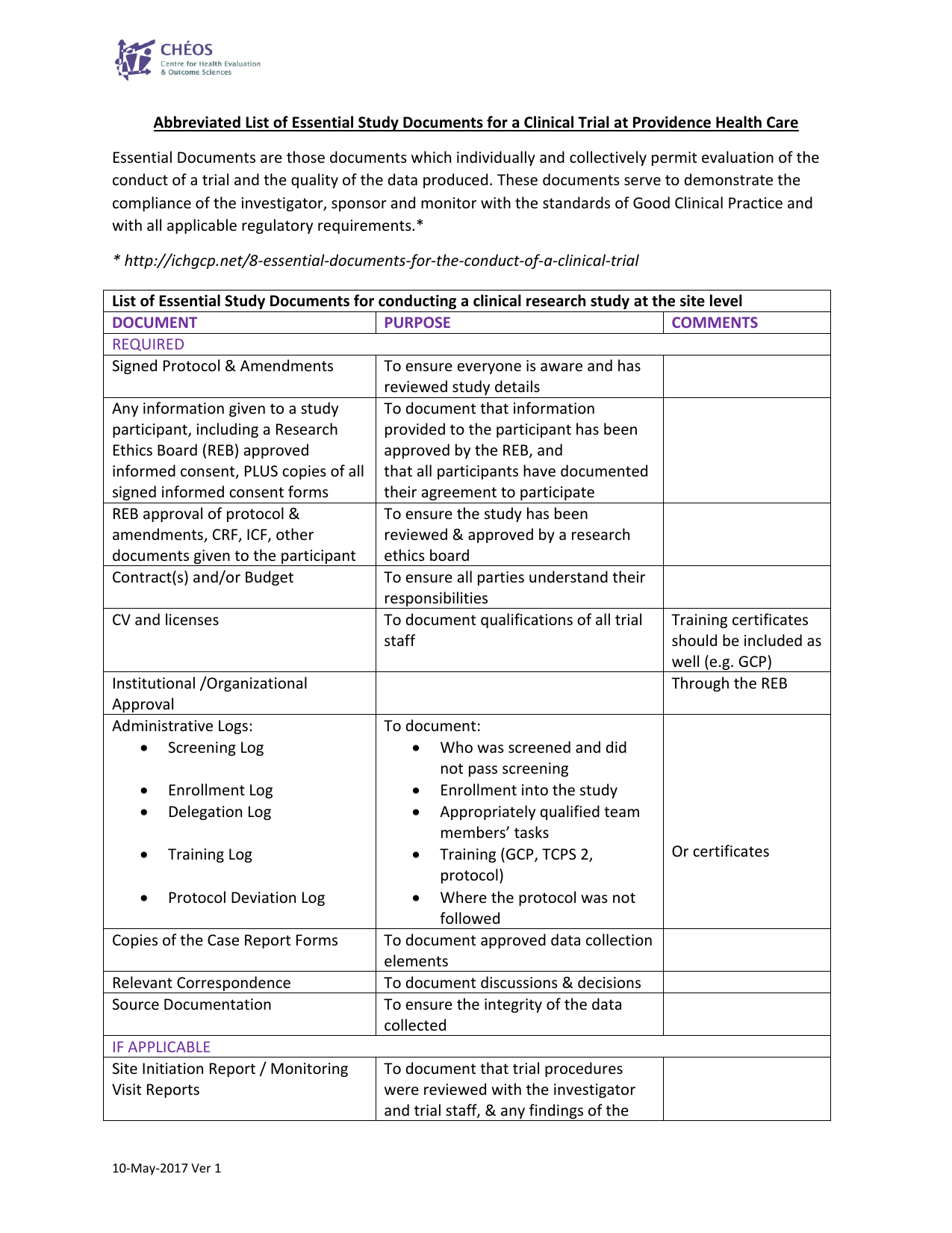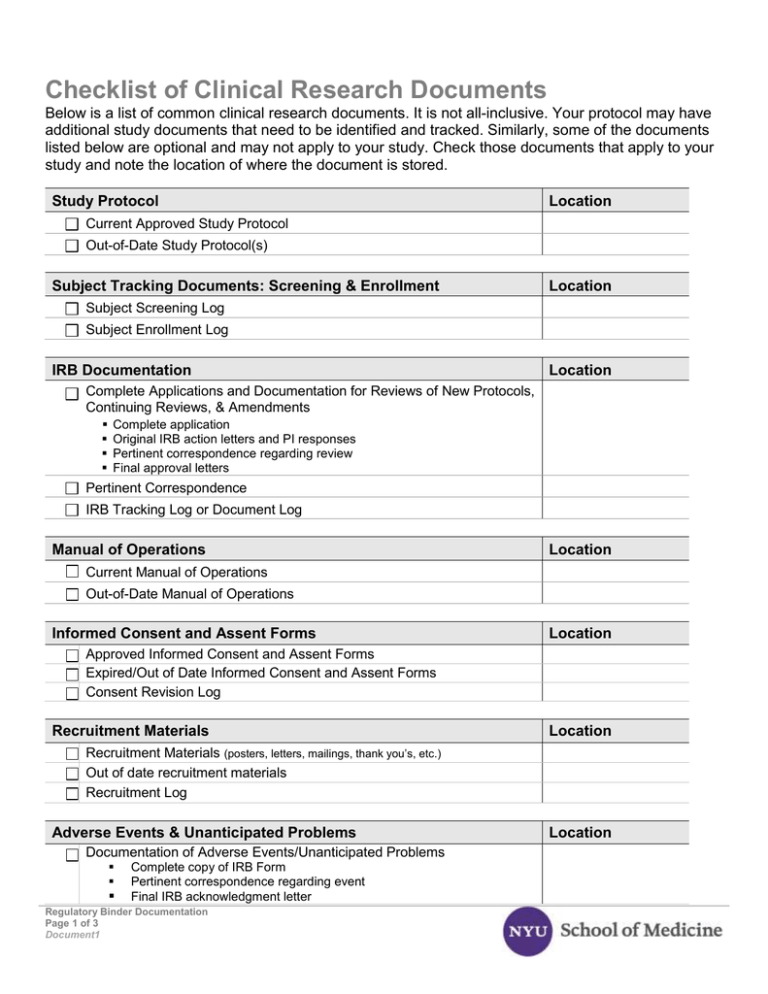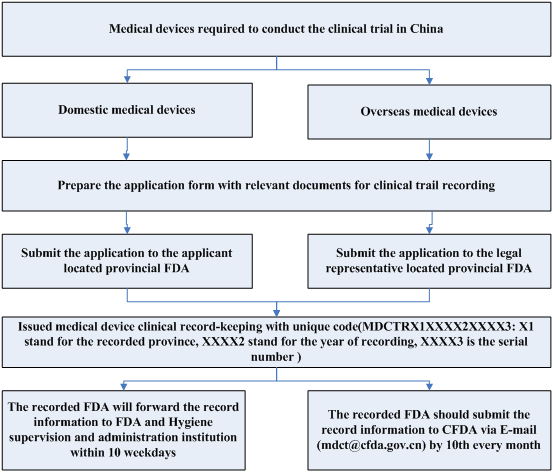
Clinical trial activities required to be recorded in China local FDA - Regulatory News - Medical Devices - CIRS Group

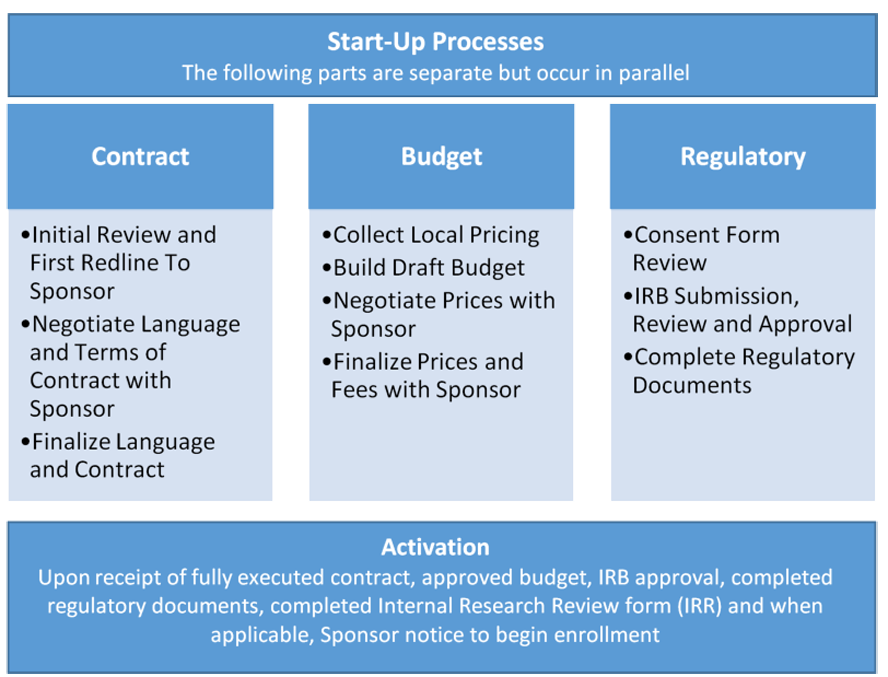
CLINICAL TRIALS - Spotlight on Quality in Study Startup: Automated Workflows Encourage Upfront Planning & Downstream Improvements in the eTMF

What you Need and When – The Key Documents in the Drug Lifecycle - Trilogy Writing & Consulting GmbH

Medical writing for regulatory submission in clinical research and its challenges pdf2 by Medical Writing Experts - Issuu